Off-label, But Lucrative
By Nav Dhillon
Big pharma profits when doctors prescribe a drug for a disorder other than the one that earned FDA approval. The practice ranges from evidence-based to highly egregious.
One in five prescriptions written in the United States is for a drug that hasn’t been approved for what’s ailing the patient.
That factoid from the Agency for Healthcare Research and Quality website casts a shadow of doubt over many of the prescriptions known as “off-label.”
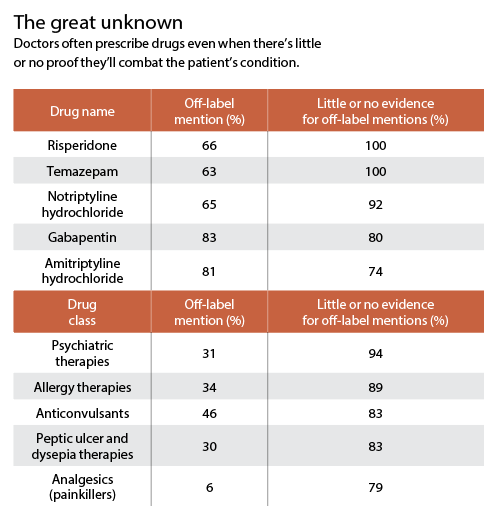
It’s when doctors prescribe a drug for a disorder other than the one that earned Food and Drug Administration (FDA) approval. They might, for example, order a diabetes drug for a patient who’s not diabetic but wants to lose weight.
Off-label prescribing is often appropriate, and...
Topics
-
How Costco and Walmart Maintain Growth—Even During Economic downturns
|Companies with extremely loyal customers tend to grow their revenue significantly faster than their less popular counterparts -
The Sahm Rule’s Red Alert: Will the Fed Hit the Panic Button?
|Two successful methods of predicting recessions are issuing warning signs -
A New Player in Products for All Ages
By James Melton
|No longer part of Johnson & Johnson, Kenvue blazes a new trail for its brands like Band-Aid and Listerine -
Death, Taxes … Healthcare Inflation
|Customer lifetime value in the health insurance sector has a hidden advantage -
Q2 Earnings: Expectations Run High, But Headwinds Are Building
|Big banks lead off earnings season, as investors look for clues as to whether the bull run will continue -
Costco’s Behemoth Private Label
By James Melton
|The warehouse club’s Kirkland Signature brand produces more revenue than Coca-Cola -
Cradle-to-Grave Customers
By Ed McKinley
|Get them when they’re young and keep them forever. It’s a (mostly) winning formula. -
Want to Grow CLV? Start Kids Early.
|A movement is arising to protect younger users from the alleged dangers of social media -
Another U.S. Credit Downgrade Appears Likely
|No matter who wins the election, lawmakers should act quickly to halt deficit spending -
What France’s Election Means for Global Credit Spreads
|In the summer of 2024, political uncertainty in Europe is becoming a key focus on Wall Street -
It’s a ‘Goldilocks Rally,’ But Will it Last?
|Recession poses the greatest threat to the markets’ current record highs -
The FDIC’s Got 63 Problems, and These Banks Are Them
|There are now 63 financial institutions on the FDIC’s Problem Bank List -
First Solar (FSLR): Where Tariffs, Subsidies and AI Collide
|New tariffs levied against China could benefit American manufacturers of solar cells, but there are drawbacks as well -
Green Shoots Appear to be Sprouting in the U.S. Bond Market
|The bond market has shown signs of a revival, reflecting increasing optimism about a potential rate cut. -
Market Volatility Hits Chinese Stocks, but PDD Holdings (PDD) Continues to Climb
|PDD Holdings reported impressive Q1 earnings, but the company’s valuation remains depressed due to continuing geopolitical risks -
OpenAI’s Sam Altman Fuels Oklo’s Public Debut with SPAC Merger
|Oklo, which recently debuted on the New York Stock Exchange via a SPAC merger, aims to build on previous nuclear energy innovations by producing small-scale reactors, known as SMRs -
How a Resurgence of the U.S.-China Trade War Could Disrupt the Economy and Stock Market
|On the campaign trail, Trump says he plans to raise import duties on many of the country’s trading partners if he’s re-elected to the White House. That could be disruptive… -
Intel’s Revamped Foundry Division is a Rising Threat to Taiwan Semiconductor
|Intel’s revamped foundry division wants to take on industry leader Taiwan Semiconductor—and appears to be making progress -
Another Regional Bank Just Failed, Here’s What You Need to Know
|After the latest bank failure, government regulators may need to help ease the stress in the financial system