What You Should Know About Coronavirus Testing and Antibodies
COVID-19 was only first discovered in late 2019, meaning research into the makeup of the virus and its effect on humanity is in the very early stages.
As a result, the most urgent COVID-related priorities are testing (identifying, isolating, and tracing outbreaks), treatment (medical/pharmaceutical intervention for the infected) and vaccine development (preventative intervention).
In a recent article, Luckbox examined the current race toward a COVID-19 vaccine.
Today, the focus shifts to coronavirus testing—the method by which infected individuals (past and present) are identified.
Because individuals contracting COVID-19 tend to exhibit symptoms similar to other human afflictions (i.e. the cold, the flu, etc.), it was necessary to specially design tests to evaluate whether a person has (or has had) the virus.
When it comes to coronavirus testing, there are therefore a couple of critical items to keep in mind:
- The type of test in question
- The stage of the disease associated with each type of test
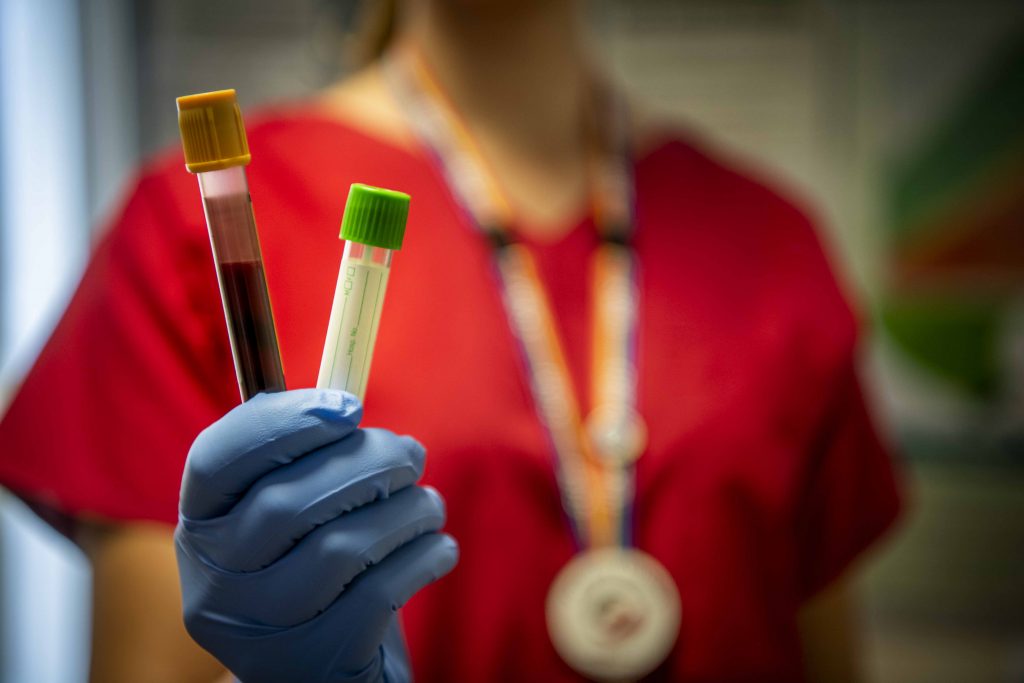
For example, one testing buzzword heard routinely in the media these days is the “antibody test.” Anyone hearing the words “antibody test” should immediately associate them with the more simplified “blood test.”
An antibody test is simply a blood test that screens for COVID-19 antibodies. The presence of such antibodies in a person’s blood indicates whether they were likely exposed to the coronavirus because the human body produces unique antibodies in response to infectious agents.
It generally takes four to seven days for the human body to produce such antibodies, meaning people in the early stages of a coronavirus infection won’t necessarily test “positive” to this form of test. There are two types of antibodies that may appear in a test such as this:
- IgM antibodies: likely to develop during the early stages of an infection
- IgG antibodies: more likely to develop after an individual has recovered
In terms of quality and accuracy, people should keep in mind that there are more than 100 different varieties of COVID-19 antibody tests in the United States alone. The Food and Drug Administration (FDA) has allowed them to be marketed without authorization, which means quality and accuracy can vary.
One bonus for antibody tests is their associated speed in processing. It only takes a few minutes to test blood for COVID-19 antibodies, but it should be noted that it is not yet known for certain whether the presence of antibodies means that an individual will be protected from future infection.
The second type of test that people need to be aware of is the simple diagnostic test for COVID-19, which is often referred to by its not-so-simple name: a PCR (polymerase chain reaction) test.
PCR tests screen for the presence of the genetic material associated with the coronavirus. PCR tests are conducted using mucus samples from a person’s mouth or nose. PCR tests are therefore less invasive than blood tests.
PCR tests are highly accurate and don’t require the body to have produced antibodies to return a “positive” result.
The one limitation with PCR tests is that it can sometimes take two to three days for the coronavirus to start replicating in a person’s nose and throat, which means a recently infected individual could still produce a “negative” PCR test.
PCR tests are often sent to centralized laboratories for screening, which means the roundtrip timeline of a PCR test can be as long as three days. If a testing facility already has the required diagnostic equipment on site, a PCR test may take as little as 15 minutes.
PCR tests are commonly used on individuals believed to have a “current” infection, while antibody tests are conducted on those who may have been infected previously.
The third type of COVID-19 test to be aware of is the so-called “antigen test.”
Antigens are foreign substances, like bacteria or viruses, that induce immune responses in the body.
The antigen test is intended to fill the gap left by the other two tests (antibody and PCR) because antigens can present immediately, whereas the biological material detected in the other two tests may not be present for the first several days after infection, or longer in the case of the antibody test.
An antigen test is conducted using secretions from a patient’s nose and throat (much like the PCR test), but in this case, the sample is screened for the presence of a specific foreign invader through the identification of unique proteins.
Antigen tests are the newest of the three test types on the market. The first antigen test in the United States gained FDA approval on May 8.
The company that developed the first-approved antigen test in the U.S. was Quidel Corporation (QDEL).
Quidel Corporation is known for developing, manufacturing and marketing diagnostic testing solutions for applications in infectious diseases, cardiology, thyroid, women’s and general health, eye health, gastrointestinal diseases, and toxicology globally.
It should be noted that the accuracy associated with antigen tests isn’t yet fully understood. Antigen tests have historically been highly accurate when testing for “strep throat” (aka streptococcal pharyngitis), but less reliable when testing for influenza (i.e. the flu).
Whether an individual can get tested, and under what circumstances, varies greatly by location.
Many cities in the United States offer free testing sites, which can be utilized by individuals who believe they may have an active infection, or by individuals who believe they may have been infected previously.
Generally speaking, individuals seeking medical attention for flu-like symptoms, or other common symptoms associated with the coronavirus, will be automatically tested at the clinic or hospital where they seek assistance.
Listed below are some of the most prominent publicly traded companies currently involved in COVID-19 testing:
- Abbott Labs (ABT)
- Bayer (OTC: BAYRY)
- Becton Dickinson (BDX)
- BioMerieux (OTC: BMXMF)
- Co-Diagnostics (CODX)
- Danaher (DHR)
- Eli Lilly (LLY)
- Grifols (GRFS)
- LabCorp (LH)
- OPKO Health (OPK)
- Quest Diagnostics (DGX)
- Quidel (QDEL)
- Roche Holdings (OTC: RHHBY)
- Thermo Fisher Scientific (TMO)
For more information on the race toward a COVID-19 vaccine, readers may want to review this previous Luckbox article when scheduling allows.
For a broader update on the biotechnology sector, a recent episode of Tasty Extras on the tastytrade financial network may also be of interest.
Sage Anderson is a pseudonym. The contributor has an extensive background in trading equity derivatives and managing volatility-based portfolios as a former prop trading firm employee. The contributor is not an employee of luckbox, tastytrade or any affiliated companies. Readers can direct questions about any of the topics covered in this blog post, or any other trading-related subject, to support@luckboxmagazine.com.